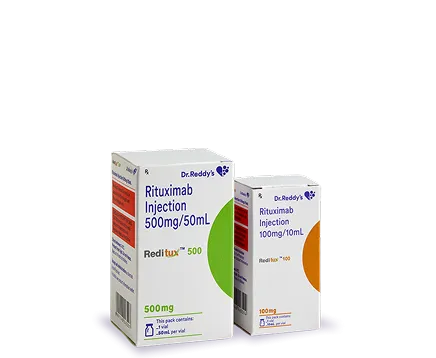
What is Reditux?
Reditux, a biosimilar rituximab - the world’s first monoclonal antibody biosimilar was launched by Dr. Reddy’s in 2007. It is used to treat Non-Hodgkin’s Lymphoma, Chronic Lymphocytic Leukaemia and Rheumatoid Arthritis. This monoclonal antibody has helped over half a million patients worldwide.
Storage Conditions
2-8 degrees Celsius (refrigerate), Do not freeze.

Presentation
Vial containing concentrate for solution for infusion.

Route of Administration
Intravenous (IV) (upon prescription only and administered by a healthcare provider.)


Indications
How Rituximab works?
Mechanism of Action
Rituximab is a chimeric murine/human monoclonal immunoglobulin G1 antibody that targets CD20, which is a B-cell differentiation marker. CD20 is a cell surface marker specifically found on late pre-B and mature B lymphocytes and is not found on other cell types or free in circulation. The only binding site for Rituximab is CD20 on B-cells. The binding of Rituximab to cell surface CD20 located on the B lymphocytes results in the destruction of the lymphocyte by three potential mechanisms: complement-dependent cytotoxicity, stimulation of apoptosis, and antibody-dependent cytotoxicity.
We Meet Global Standards
- Publication
- 23-04-2025
Abstract No: 0556, ISNCON Conference 2014 Efficacy and Safety of Indigenously Developed Darbepoetin Alfa in Dialysis Patients - A Randomised Study
- News
- 07-06-2025
Alvotech and Dr. Reddy’s to co-develop biosimilar candidate to Keytruda® (pembrolizumab)
- Publication
- 12-02-2020
Pharmacokinetic Similarity and Comparative Pharmacodynamics, Safety, Efficacy, and Immunogenicity of DRL_RI Versus ReferenceRituximab in Biologics‑Naïve Patients with Moderate‑to‑SevereRheumatoid Arthritis: A Double‑Blind, Randomized, Three‑Arm Study
- Publication
- 06-12-2019