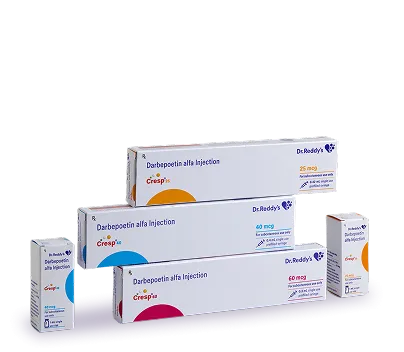
What is Cresp?
Cresp is a long-acting biosimilar of darbepoetin alfa, developed by Dr. Reddy’s Biologics, used to treat anaemia by stimulating red blood cell production. It is indicated for patients with chronic kidney disease (CKD) and chemotherapy-induced anaemia, helping to manage symptoms and reduce the need for transfusions.
Storage Conditions
2-8 degrees Celsius (refrigerate), Do not freeze.

Presentation
Vial/PFS containing drug

Route of Administration
Subcutaneous (SC) (upon prescription only).


Indications
How Darbepoetin Alfa works?
Mechanism of Action
Cresp works by stimulating red blood cell production through its active ingredient, darbepoetin alfa, a long-acting erythropoiesis-stimulating agent (ESA). It binds to erythropoietin receptors on progenitor cells in the bone marrow, activating the JAK-STAT signaling pathway, which promotes the proliferation and differentiation of these cells into mature red blood cells. The addition of extra carbohydrate chains in darbepoetin alfa prolongs its half-life, allowing for sustained activity and less frequent dosing compared to traditional erythropoietin. This extended duration of action makes it a convenient and effective option for managing anemia in chronic kidney disease and chemotherapy patients.
We Meet Global Standards
- News
- 17-12-2023
Dr. Reddy's, Coya Therapeutics Ink Licensing Agreement For ALS Treatment Drug
- News
- 06-12-2023
Coya taps familiar friend Dr. Reddy's to commercialize ALS med
- News
- 29-07-2024
Dr. Reddy’s biosimilar gets recommendation from European Medicines Agency
- News
- 29-07-2024