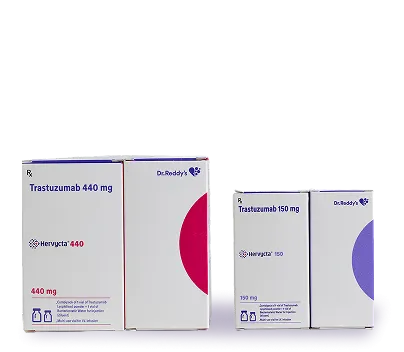
What is Hervycta?
Hervycta is the brand name for Trastuzumab, a biosimilar of the reference product Herceptin. It is primarily used in the treatment of HER2-positive cancers. Hervycta is designed to deliver comparable efficacy and safety to the reference product, offering a cost-effective option to improve access to advanced cancer therapies.
Storage Conditions
2-8 degrees Celsius (refrigerate),Do not freeze.

Presentation
Vial containing lyophilised powder for infusion + Bacteriostatic water for diluent.

Route of Administration
Intravenous (IV) (upon prescription only and administered by a healthcare provider.)


Indications
How Trastuzumab works?
Mechanism of Action
Trastuzumab is a monoclonal antibody that targets the HER2/neu receptor, which is overexpressed on the surface of certain cancer cells, particularly in HER2-positive breast cancer. It works by binding to the extracellular domain of the HER2 receptor, inhibiting the signaling pathways that promote cell proliferation and survival. Additionally, trastuzumab activates antibody-dependent cellular cytotoxicity (ADCC), a process where immune cells are recruited to destroy the HER2-positive cancer cells. This dual action - blocking HER2 signalling and enhancing immune cell-mediated destruction -helps to reduce tumour growth and is the basis for trastuzumab’s therapeutic efficacy in treating HER2-positive cancers.
We Meet Global Standards
- Publication
- 21-12-2020
Safety and tolerability of Peg‑grafeel, a pegfilgrastim, for the prophylactic treatment of chemotherapy‑induced neutropenia and febrile neutropenia: A prospective, observational, postmarketing surveillance study in India
- Publication
- 09-10-2013
A Randomized, Multi Centre, Open-Label Study to Evaluate the Efficacy and Safety of Peg G-CSF as Compared to Grafeel in the Prophylaxis of Severe Neutropenia in Cancer Patients Receiving Cytotoxic Chemotherapy
- Publication
- 31-03-2025
Abstract No: 0556, ISNCON Conference 2014 A Randomized, Comparative, Open-label Clinical Trial of Darbepoetin Alfa Biosimilar for the Treatment of Anaemia in Pre-Dialysis Chronic Kidney Disease Patients
- Publication
- 23-04-2025